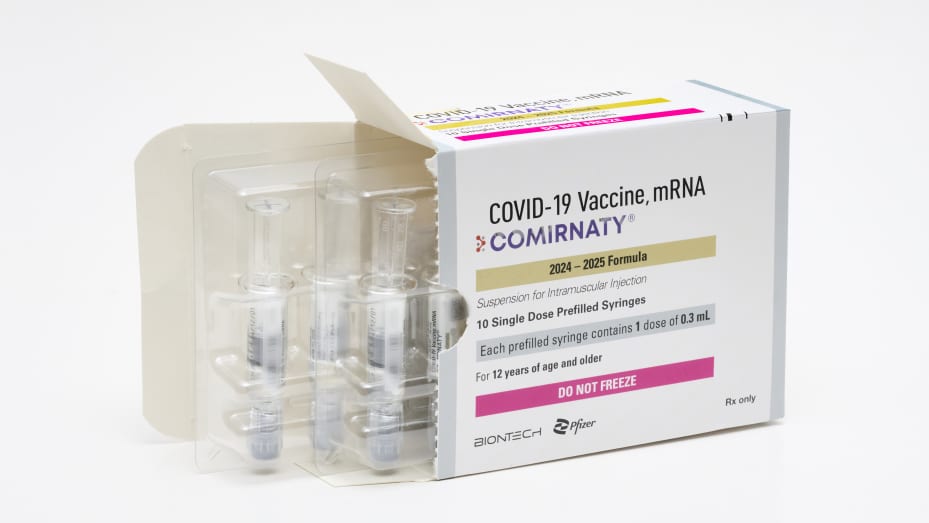
The Food and Drug Administration has recently approved new COVID-19 vaccines from Pfizer and Moderna, with both companies stating that the vaccines will be available in the coming days ahead of the fall and winter respiratory virus surge. The shots target the KP.2 COVID-19 variant, which is a descendant of the omicron subvariant that was predominant in the U.S. earlier this year.
The FDA granted accelerated use authorization to Moderna and Pfizer’s updated vaccines to better protect against the serious consequences of COVID-19 by targeting currently circulating variants. Moderna expects its vaccine to be available in the coming days, while Pfizer has stated that its shot will be available immediately following approval.
Initially, the FDA asked vaccine manufacturers to create shots that target the JN.1 variant of the coronavirus, but later changed this recommendation to focus on the KP.2 strain of the JN line after reviewing updated case data.
Novavax, which submitted a vaccine targeting the JN.1 variant for approval, did not receive FDA approval on Thursday. The company stated that it is working with the FDA to complete its review and expects authorization to happen in time for peak vaccination season.
Source: https://www.pressrundown.com/coronavirus/fda-approves-updated-covid-vaccines-from-moderna-and-pfizer